Polymer injection for treatment and prevention of endoleaks
This project was aimed at obtaining clinically relevant insights into several aspects of a novel polymer injection technique for treatment and prevention of complications after endovascular repair of abdominal aneurysms.
An abdominal aortic aneurysm is a widening of the main artery at the height of the abdomen. It is typically treated by endovascular placement of a stent graft to guide the blood flow and relieve pressure from the weakened vessel wall. However, due to potential complications after this procedure, mainly leakage of blood into the aneurysm, lifelong monitoring is needed and reinterventions are often required. These leakages could be prevented by filling the whole aneurysm with an injectable polymer, essentially plugging potential leaks in advance.
The novel polymer technique developed by TripleMed is specifically designed for this. However, important questions remain before this technique can be safely used in clinical practice. The aim of this project was to increase the knowledge about the visibility of the polymer and mechanical interaction between the polymer and the stent graft. These aspects are of utmost importance with regard to safety and durability of treatment.
Several aspects were investigated through the use of clinically relevant, physical models of the abdominal aorta, which were designed and developed in-house (Figure 1). These models were used to investigate imaging techniques, both during injection of the polymer and during follow-up after the treatment (Figure 2). It was found that the contrast in the polymer can be reduced by ~33%, reducing artifacts on computed tomography (CT) typically used for follow-up, while retaining sufficient detectability for safe injection. It was also found that ultrasound, another commonly used follow-up technique, is not suitable after polymer injection because the polymer significantly weakens the measured ultrasound signals. Finally the effect of the polymer on the natural motion of the aorta and stent graft was investigated. These insights help bring the polymer technique closer to clinical practice.

Figure 1. Clinically relevant, physical models of abdominal aortic aneurysm; just the model (left), with a stent graft placed inside (middle) and with a stent graft and polymer injection (right)
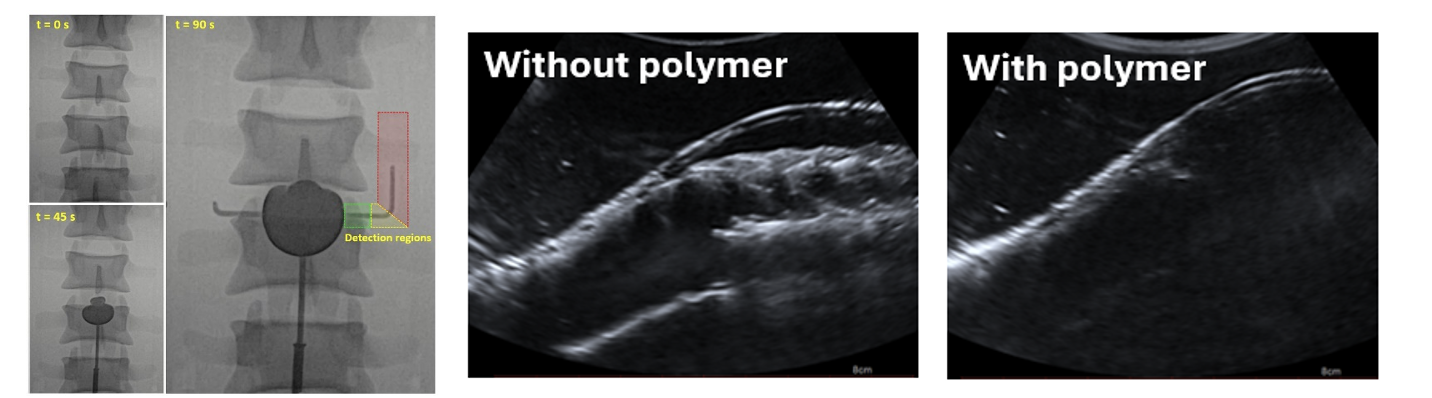
Figure 2. Imaging results of models with polymer injections; X-ray measurements during injection, at several timepoints (left) and ultrasound measurements during follow-up, without polymer, showing the vessel wall and stent graft (middle), and ultrasound measurements with polymer, only showing the top vessel wall and obscuring the stent graft and bottom wall




