Can we use the gut microbiome to improve cancer therapy?
Immune checkpoint inhibition (ICI) therapies, designed to (re)activate potent anti-tumour T-cell responses, have revolutionized cancer treatment, but not everyone benefits because of low treatment response or severe side effects. Surprisingly, the intestinal microbiota plays an important role in the success of ICI therapy, suggesting a crosstalk between the immune system and bacteria in the intestine. Similarly, the composition of the microbiota is believed to also be a key determinant for therapy toxicity. In a collaborative effort, UMCU partners, Artizan Biosciences and MicroViable Therapeutics aim to harness the microbiota to improve ICI therapy.
ICI therapy is only effective in a subset of patients and is not without risks; despite being well tolerated by some patients, ICI toxicity (“immune-related adverse events”, or irAEs) can be severe, irreversible and even fatal. The HARNESS project will positively impact the health and lifespan of a large, expanding part of the population by making existing therapy more efficient and cost-effective. As cancer has been shown to disproportionately affects lower socio-economic groups, improved therapy will also help to decrease socio-economic health inequalities.
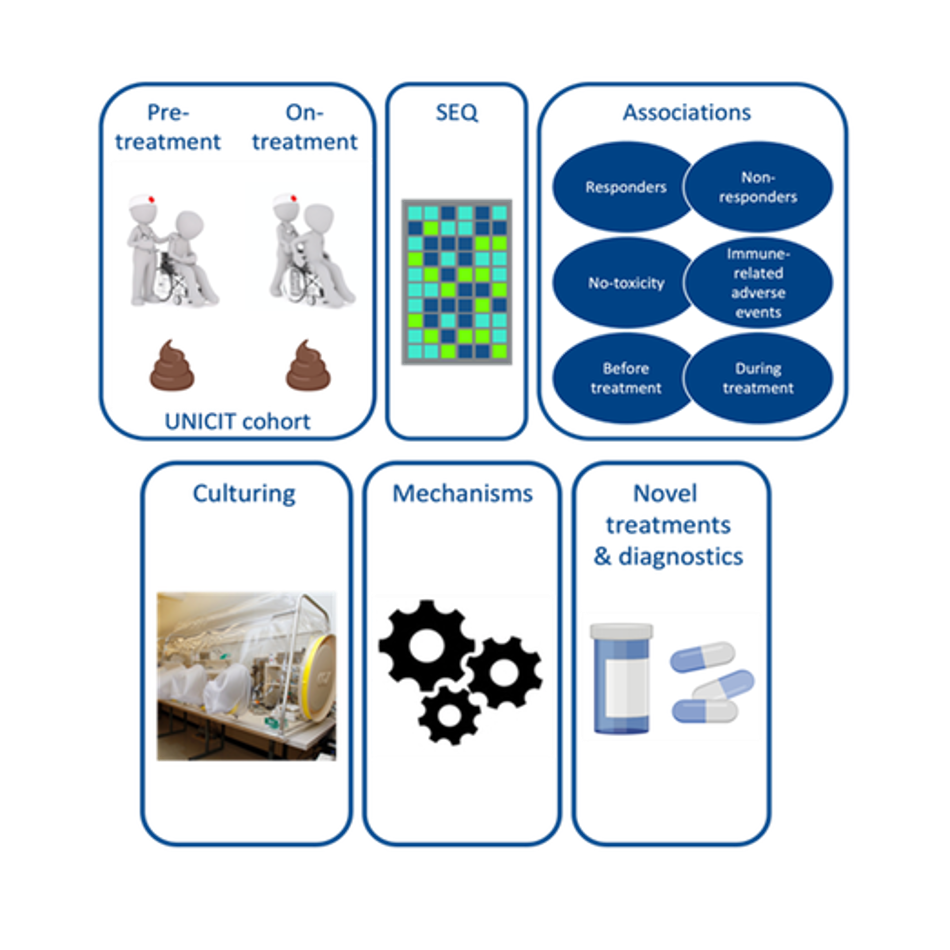
In this project, we will make use of an extensive biobank of faecal material from ~200 cancer patients before and during ICI therapy (including patients with intestinal irAEs) and i) identify pivotal bacteria that evoke immune responses using IgA-SEQ technology, ii) correlate these bacteria with intestinal irAEs and treatment response, iii) isolate bacteria- of-interest through advanced culturomics, and iv) unravel the mechanisms through which they affect ICI therapy using in vivo and in vitro model systems.
We aim to uncover these bacteria and unravel the mechanisms by which they impact treatment and intestinal irAEs, and provide a rationale for microbiota-targeted therapy that maximize effectiveness of therapy while minimalizing intestinal irAEs.