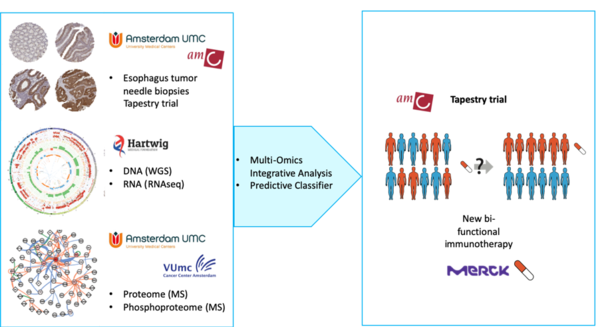
Multi-Omics response prediction for immunotherapy in esophageal cancer
Omics-Predict is a public private partnership of Amsterdam UMC, Hartwig Medical Foundation and Merck. Using global profiling at the level of DNA and its encoded gene products, the proteins and phosphoproteins, genomic aberrations will be identified and hyperactive cellular signalling pathways in esophageal cancer and candidate biomarkers that are associated with (lack of) response to bintrafusp-alfa, a new dual inhibitor of the TGFb pathway and checkpoint inhibitor.
The outcome of esophageal cancer is poor, with an overall 5-year survival rate of 10% worldwide. For patients with medically or technically inoperable esophageal cancer the treatment of choice is definitive chemoradiation. Thus far, no targeted treatments are available for this patient population. In the clinical TAPEStry study the aim is to improve treatment outcome by combining chemoradiation with a novel TGFb-PDL-1 inhibitor.
In this project, the need for novel diagnostic strategies will be addressed that accurately predict which patient will benefit from the novel bi-functional immunotherapy treatment. To this end, tumor needle biopsies in the TAPEStry trial (WP1) will be initiated and collected and the power of mass spectrometry-based (phospho)proteomics (WP2) will be combined with whole genome and whole transcriptome sequencing (WP3) to improve the understanding of drug bintrafusp-alfa and predict which patients will benefit from this innovative dual inhibitor. To this end, tumor needle biopsies will be analysed using state-of-the-art workflows at VUmc and Hartwig Medical Foundation. Data will be correlated to patient response and a predictive OMICs signature for future patient stratification will be developed (WP4).