Generation of human muscles on a chip
In this project, Erasmus MC, LUMC, and the company Optics11 B.V. collaborated to develop technology that enables the generation and functional assessment of human muscles grown on a chip. In particular, the technology allows assessment of multiple muscles at the same time, allowing the testing of the response to therapies.
There are over 1,000 neuromuscular disorders but only for very few treatment is available. A major reason is the lack of suitable model systems for human muscle disease. This leads to failed clinical trials, the need of expensive care, and impaired contribution to workforce. The effects are negative both for society (large impact on family life) and the economy (failure for drug developing companies, expensive care, inability to work). It is therefore vital to develop models for human muscle disease and technology that can recapitulate the disease in vitro and that can be used to test novel treatments.
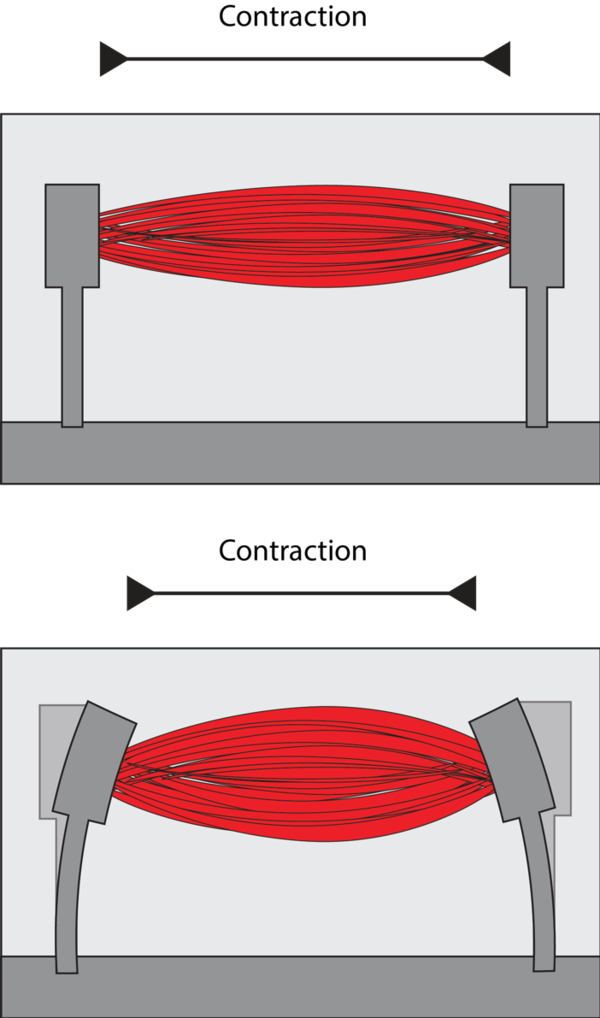
We have developed muscle-on-chip technology that allows the generation of human muscles from any individual in the laboratory. These muscles are grown in 3 dimensions on a chip, and they are able to contract upon stimulation, just like a real muscle. We have developed a device that can handle multiple muscles at the same time. Because this technology more faithfully represents the disease processes and can now be applied in semi high-throughput, it offers a considerable advantage compared to existing methods that rely on artificial cells or animals, and is expected to result in faster development of treatments.
The work has led to a device that has parallel measurements of contractile force. It has been applied to two muscle disorders, Pompe disease and FSHD, and this research has resulted in the identification of novel potential targets for therapy.