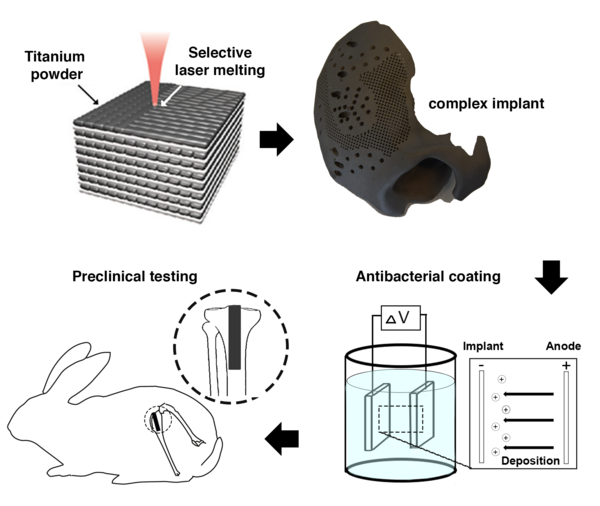
Bactericidal Coating on Complex Titanium Implants
Bactericidal Coating on Complex Titanium Implants (BCCTI) is conclusion of a recently successful partnership of Department Orthopedics-UMC Utrecht and Amber Implants to tackle one of the most important challenges of orthopedic surgery: Implant-associated infections (IAIs).
IAIs occur in approximately 1-2% of all primary (first) implant surgeries and up to 8% of revision surgeries. IAIs can be devastating for patients, with long hospitalisation, high costs, and extensive operative procedures to remove the biofilm and replace the implant. In the Netherlands, the number of these infected implants are estimated at 1500 per year, with an annual hospitalisation cost of approximately €75 million.
In this project, a bactericidal coating has been developed for 3D-printed titanium implants based on electrophoretic deposition coating technology. The surface properties, antibiotic release profiles and antibacterial properties of the developed coating have been investigated and verified that the coating works well in principle under laboratory conditions. Subsequently, a special 3D-printed implant was designed for a bone defect in a rat that does not heal on its own. Ethical approval has been obtained to conduct an animal study in which a bacterial infection can be reproducibly induced in a rat and where the optimal doses of an antibiotic in the coating can be determined. This infection model has been tested in a first series of animal experiments. The effectiveness of the coating will be tested in a follow-up experiment.