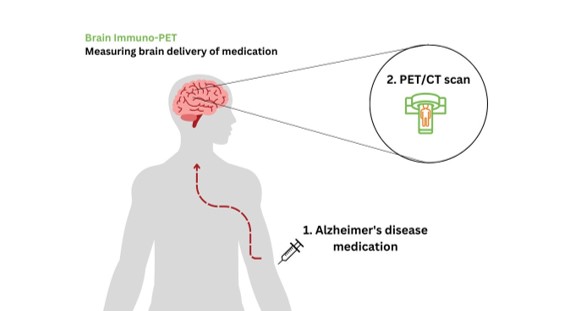
Measuring brain delivery of novel medication for Alzheimer’s Disease
This proof-of-concept study develops the technique to quantify brain delivery and distribution of monoclonal antibodies (mAb) with 89Zr-immuno-PET. The partnership between BV Cyclotron, global leader of 89Zr production, and Amsterdam UMC, developer of the technique for stable 89Zr-labelling of mAbs for brain targets, together with the use of a highly sensitive PET/CT system at the Amsterdam UMC, will allow us to quantify mAb delivery and distribution in the brain.
The current landscape of Alzheimer’s disease drug development is rapidly changing with the advent of anti-amyloid mAbs. However, some clinical trials show lack of effect possibly due to low drug delivery to the brain, since the blood brain barrier mostly prevents these large molecules from entering. The exact brain uptake is unknown, but the estimated brain delivery is ~1%. This project will for the first time measure in humans how much anti-amyloid mAb medication is delivered to the brain.
In this project, we will label anti-amyloid mAbs with 89Zr. This will allow us to visualize and quantify the brain delivery of these mAbs in Alzheimer’s disease patients with PET imaging. In addition, we will assess how the brain uptake is related to the distribution of amyloid pathology in the brain of Alzheimer’s disease patients. This project will open up a new field of research for studying mAbs (and other types of medication) in the brain. This will significantly contribute to improving the development of these medications and of techniques that enhance the brain delivery.
At the end of the project, we will have developed the immuno-PET method (including tracer production, optimized study design and analysis methods) for brain targets in humans. In addition, we will have quantified for the first time the brain delivery and distribution of an anti-amyloid mAb in Alzheimer’s disease patients.