Improved drugs by selecting safer drug targets with TargetTri
In this project, TNO and its partners – Lundbeck, Leo Pharma, Novo Nordisk and AstraZeneca – joined forces to design and develop a web-based knowledge platform that answers one of the key questions in preclinical drug discovery: what are the toxicological risks associated with the envisaged drug target? The project fully embraced the knowledge and experience of the pharmaceutical partners on preclinical toxicology in general, and target-related safety issues in particular. Combined with TNO’s multi-disciplinary experience in building and exploiting knowledge-based platforms, the collaboration set the optimal stage for the development of the target triaging platform TargetTri.
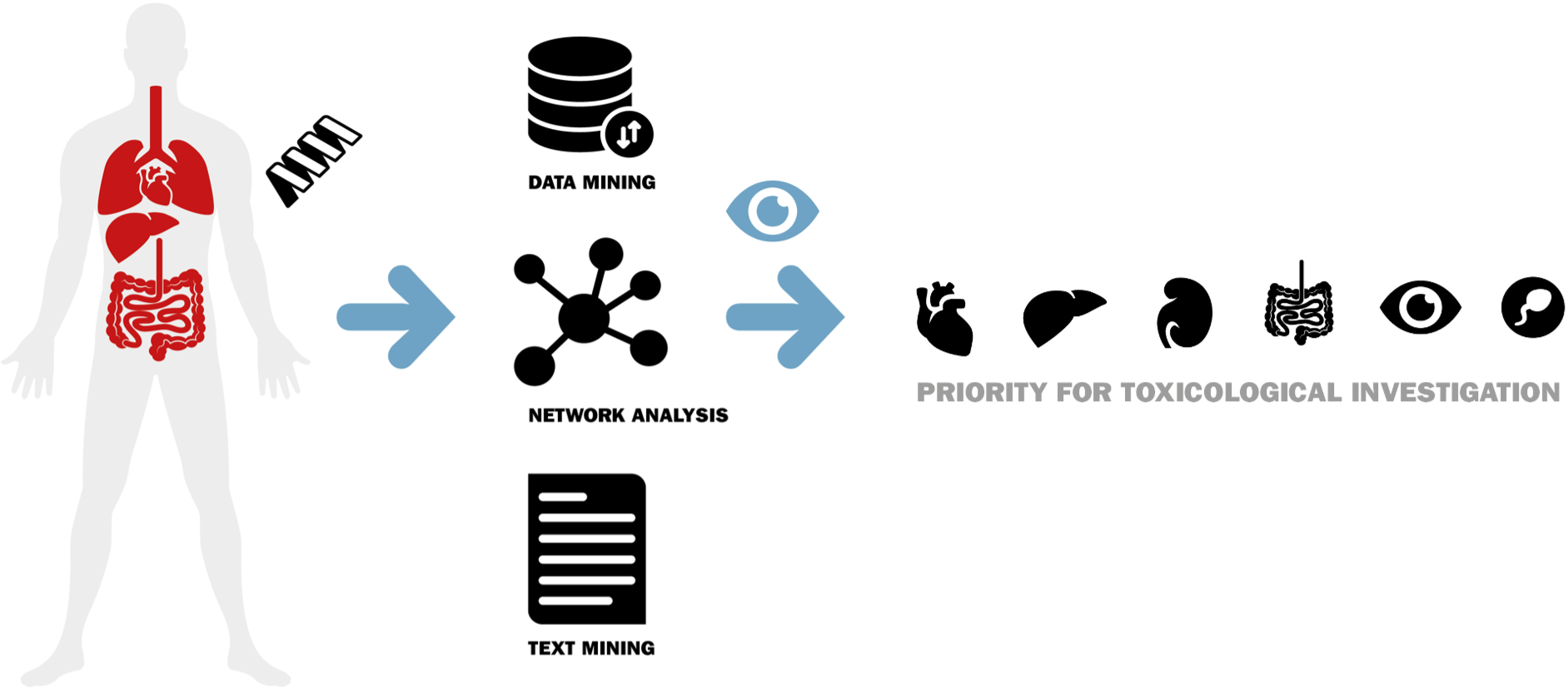
Identifying safety issues that could arise from drug target modulation at the very start of the development pipeline is important to sustain the efficient and successful development of novel drugs. Such target safety assessments (TSAs) require the integration of large amounts of data, collected and analyzed by a variety of techniques. To make these mostly hand-made target-centric safety assessments more efficiently and accurate, this project designed and built a web-based platform for semi-automated drug target triaging.
The activities encompassed an initial definition (blueprint) of the content and functionalities of the TargetTri system, and an iterative implementation and optimization in concert with the project partners. The project also included fundamental research on data integration, text-mining strategies and prediction models to enable efficient and accurate TSAs based on current knowledge and state-of-the-art techniques.
The project delivered a benchmarked TargetTri system that has, in part, reached TRL6. It is currently being used by the partners for toxicological research of drug targets. TargetTri has been found particularly useful by the partners to explore new targets and obtain a first view on the toxicological aspects of target modulation. The integration of data sources makes information collection and data interpretation considerably more efficient, as does the text-mining. The collection of comprehensive ontologies, handling of big data and interpretation of abbreviations in text-mining have proven particularly challenging. The latter is essential in reducing noise in the results set and its implementation adds a unique aspect to TargetTri. Although further improvement and functionalities are warranted, the platform has demonstrated its value in the pharmaceutical industry. Further development in follow-up projects currently involve reporting, collecting drug information (compound module) and automated data interpretation, for example, showing toxicological high-lights for a target based structured data. In addition, TargetTri’s text-mined data is used for target discovery purposes.