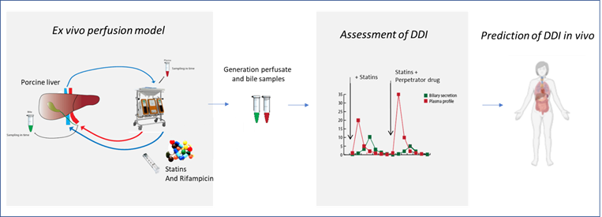
Ex vivo porcine liver perfusion to study drug pharmacokinetics
There is a lack of translational preclinical models that can predict hepatic handling of drugs, including the prediction of biliary excretion. The aim of this study was to apply normothermic machine perfusion (NMP) of porcine livers as a novel ex vivo model to predict hepatic clearance, biliary excretion and plasma exposure of statins, and the effect of drug-drug interactions (DDI) on these processes. In this project, the consortium demonstrates the applicability of NMP of porcine livers as novel preclinical model to study hepatic handling of drugs and the effect of DDI on plasma exposure and biliary excretion.
The prediction of the hepatic handling of drugs is of major importance for the pharmaceutical industry. Proper prediction of a drugs PK and the possibility of DDI can shorten the drug development process which has a major societal and economic impact.
In this project, the consortium studied the applicability of a pressure driven perfusion device as a method to predict hepatic handling of drugs. They have chosen model compounds with known PK and which are prone to interaction with other drugs. The project showed that the obtained results are in line with in vivo data, indicating the potential values of this new ex vivo model in drug development
The findings obtained in this project will be summarised in a manuscript.