Hereditary hemorrhagic Telangiectasia treatment through drug repositioning
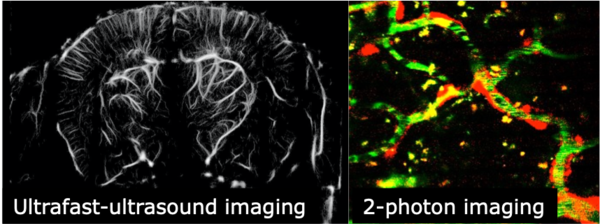
Hereditary Haemorrhagic Telangiectasia (HHT) is an inherited disease characterized by vascular malformations that affects 1 in 5000 people worldwide. Clinical manifestations include severe and recurrent nose and gastrointestinal bleeding, iron deficiency and anaemia due to fragile blood vessels. Large arteriovenous malformations are found in brain, lungs and liver and are associated with life threatening complications notably stroke, abscess and high-output heart failure. Patients with HHT have such poor quality of life that they are unable to work, require significant psychological support and are unable to maintain a normal social life. Importantly, there are 50-60 HHT patients in the Netherlands without further treatment options from a total of 1400 in 160 families known to the HHT expertise Centre. Identification of the causative gene mutations and the generation of preclinical models have revealed that disruption of cell signalling pathways regulating angiogenesis (blood vessel growth from a pre-existing vascular bed) underlie these vascular malformations indicating that drugs inhibiting angiogenesis may be effective to treat HHT. The consortium consisting of Leiden University Medical Centre and Vaderis Therapeutics, a clinical stage biotechnology company focusing on the treatment of rare diseases caused by vascular malformations have joined their forces to develop new treatment options through the development of AKT inhibitors. In this project, we have identified key disease mechanisms notably we have demonstrated that HHT gene mutations lead to a hyperactivity of AKT, one important protein of the Vascular Endothelial Growth Factor pathway regulating vessel growth. We have also developed tools and ultrasound imaging modalities allowing us to follow disease progression and drug action. We have tested various AKT inhibitors having different mechanisms of action and found that VAD044, an allosteric AKT protein inhibitor had a favourable safety profile and an excellent efficacy to inhibit AKT1/2 and the formation of vascular malformations in preclinical models of HHT. VAD044 has now entered the clinical phase of development. A phase I trial has been completed and one phase II trial (efficacy and safety in patients with HHT) is currently developed including 80 patients in 6 centres of reference of HHT. In parallel, one large European Innovation Council pathfinder proposal has been funded to implement in clinic ultrasound imaging modalities to follow drug actions in HHT.