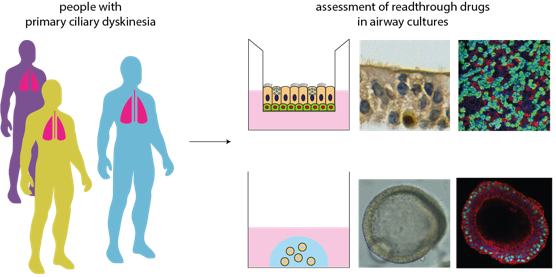
Restoring cilia function in airway cells with primary ciliary dyskinesia
This project explores whether new drugs can be developed for primary ciliary dyskinesia. The project combines candidate drugs from Eloxx Pharmaceuticals, a clinical-stage biopharmaceutical company, with expertise of the University Medical Center Utrecht to study such drugs in laboratory-cultured, patient-derived miniature airways.
All rare genetic diseases together affect up to 4% of the population in the EU. Most of these diseases remain untreatable, and are associated with significant morbidity and a shortened lifespan. About 15% of patients with genetic diseases have so-called early stop codons or nonsense mutations. An early stop codon prevents the production of a functional protein, which may cause cells to malfunction and organs to fail. Eloxx Pharmaceuticals is developing novel RNA-modulating drug candidates that are designed to treat premature stop codons. Specifically, Eloxx is developing Eukaryotic Specific Ribosome Glycoside (ESRG) modulators that promote ribosomal read-through of nonsense mutations and inhibits translation termination at in-frame premature stop codons. This results in restoration of full-length functional protein. This approach aims to directly repair the cellular defect and the genetic driver of the disease.
The University Medical Center Utrecht has developed protocols to culture miniature airways using brushed nasal cells of patients. These cultured airways are ideal to study ciliary function, which is defective in cells of patients with primary ciliary dyskinesia. The defective ciliary function in vitro will be quantified and modulation thereof will be studied in the context of treatment with Eloxx drug candidates. In the first phase, Eloxx and Utrecht hope to demonstrate that the therapeutic candidate results in robust repair of ciliary function in patient cells with stop codons. The second phase aims to address mechanism of action of the ESRG therapeutic candidate, in particular its role in nonsense mediated decay (NMD). In summary, this work will help broaden the concept of read through modalities as a treatment strategy in nonsense bearing genetic diseases and develop technologies to assist in patient stratification.