Measuring a toxic Alzheimer protein with new techniques
VUmc Neurochemistry lab and Crossbeta Biosciences have new techniques available to overcome previously encountered hurdles in development of a diagnostic tests for the earliest stage of Alzheimer’s disease. Within this project, the Neurochemistry lab and Crossbeta will combine ingredients and expertise with the aim to deliver a highly specific and highly sensitive test, targeting the earliest Alzheimer’s disease phase.
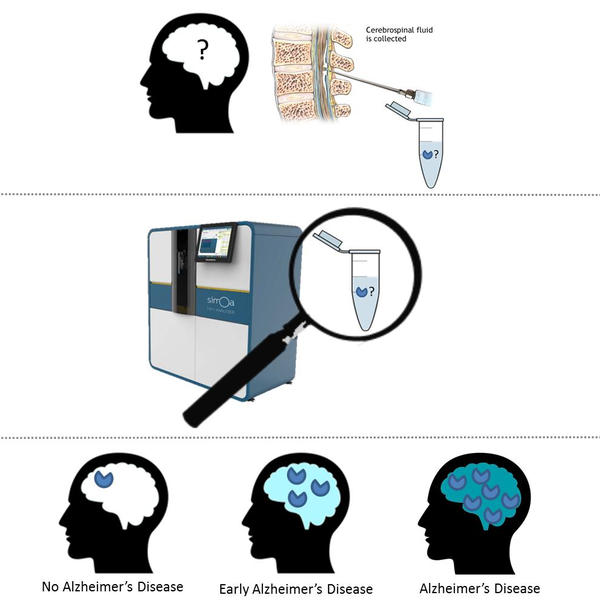
Alzheimer’s disease brain damage starts already 20 years before symptom onset. When symptoms arise, brain damage is already very extensive and irreversible. As the brain has very limited capacity for repair, we have to intervene in the disease process as early as possible. To do so, we first have to be able to diagnose patients at this earliest stage. A protein that is highly promising to use for diagnostics, is amyloid beta oligomers. The amyloid beta oligomers are amyloid beta proteins sticking together in a specific constellation. This specific form is thought to play a crucial role in cognitive deterioration in Alzheimer’s Disease.
Development of tools to measure amyloid beta oligomers was proven difficult, as these proteins are only present in very low concentrations. Also, the test should only capture this specific toxic form, and no other forms. The Neurochemistry lab has access to an advanced technical platform and expertise to measure proteins in extremely low concentrations too. Crossbeta has developed reagents that are able to specifically capture amyloid oligomers.
In this project, the consortium partners have succeeded in setting up a test to specifically measure the amyloid beta oligomers. However, they were not able to detect these oligomers in the cerebrospinal fluid. This either means that the amyloid beta oligomers are not present in the cerebrospinal fluid, or another advance in assay development technologies is needed to succeed in the precise detection of these oligomers. It remains a highly interesting and important topic in the field to develop a cerebrospinal fluid amyloid beta oligomers test. Therefore, the partners will continue to follow advances in assay development and technological improvements. When promising new methods become available, this might lead to a renewed collaboration between the consortium partners of this project.