Tool to predict target-related adverse effects of biological drugs
In this project, TNO and Genmab, joined forces to develop a systematic approach that assesses the potential safety liabilities of a biologic and its target in the first phase of drug discovery: Target Nomination. TNO’s experience in developing predictive toxicological tools was thus complemented by Genmab’s hands-on experience with human antibody therapeutics in the field of safety assessments and de-risking strategies.
Currently, 30% of licensed pharmaceutical products constitute biologics. Despite huge investments in this growing market, only about 24% of biologics that enter preclinical development are launched. One of the major factors contributing to the high attrition rate are safety issues. Identification of these issues at an early stage, however, faces major hurdles such as a general lack of suitable preclinical models. While the pharmaceutical industry has come to a general consensus in the case of small molecule drugs, safety evaluation of biologics is often approached on a case-by-case basis.
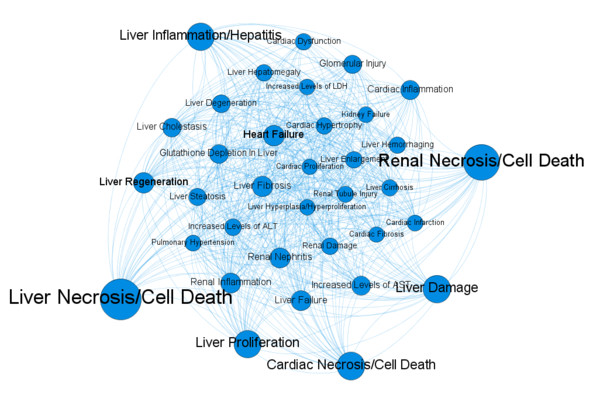
A generic safety assessment workflow was designed and developed based on the different potential causes of toxicity. Data- and text-mining methodologies were applied to identify target-, biologic-, and pathway-related effects. A predictive systems biology-based approach was applied to solve the preclinical data gaps that generally exist for biologics. Validation of the complete workflow with a novel drug target demonstrated that it is able to identify the most important adverse effects associated with drug-target interactions. Moreover, the developed systems biology approach - as a stand-alone tool - was also able to predict major toxicities. It is thus particularly useful in those cases where limited safety data is available in the public domain.
The developed workflow may serve as a blue print for early identification and mitigation of toxicological risks of drug-target combinations. Earlier termination of high-risk drug targets will reduce costs, improve efficiency, and bring innovative drugs faster to humans.
Website for more information.
Fig.1 Systems biology-based toxicity predictions of target modulation.