Effect of antidiabetic drug on brown adipose tissue in overweight men
The growing obesity epidemic has major consequences both at the individual and the socioeconomical level. The development of novel therapies for obesity is highly warranted. Brown adipose tissue (BAT) recently emerged as a novel player in energy expenditure in humans as it combusts fatty acids and glucose towards heat. Interestingly, obese subjects have less BAT activity (based on glucose uptake) as compared to lean subjects and activation of BAT, by means of intermittent cold exposure, reduces fat mass. Therefore, BAT is considered a promising novel target to reduce obesity and associated disorders. As cold exposure is not the most desired therapeutic strategy for humans, current preclinical research focuses on pharmacological activation of BAT.
 Interestingly, we have recently shown that pharmacological activation of specific receptors enhances BAT activity with respect to fatty acids uptake in preclinical studies. One of the currently used anti-diabetic drugs that enhances receptor activity is Sitagliptin. Sitagliptin reduces body weight, plasma glucose and triglyceride levels in type 2 diabetes patients. We hypothesize that sitagliptin enhances BAT activation, thereby increasing energy expenditure and combustion of some fatty acids, resulting in lowering of plasma fatty molecule levels and body weight.
Interestingly, we have recently shown that pharmacological activation of specific receptors enhances BAT activity with respect to fatty acids uptake in preclinical studies. One of the currently used anti-diabetic drugs that enhances receptor activity is Sitagliptin. Sitagliptin reduces body weight, plasma glucose and triglyceride levels in type 2 diabetes patients. We hypothesize that sitagliptin enhances BAT activation, thereby increasing energy expenditure and combustion of some fatty acids, resulting in lowering of plasma fatty molecule levels and body weight.
In a collaboration between the Leiden University Medical Center (LUMC) and Merck Sharpe & Dohme (MSD) we will perform a randomized double-blinded placebo-controlled study in 30 overweight pre-diabetic men aged 35-55 years.
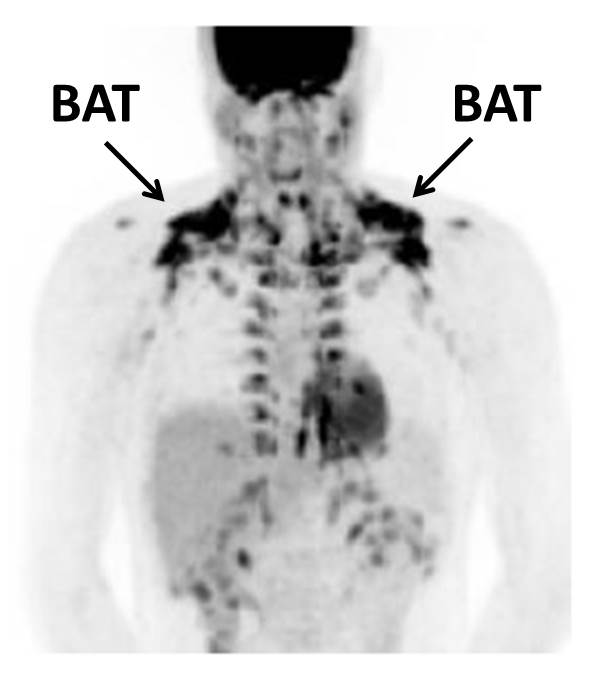
Subjects were treated for 12 weeks with Sitagliptin (100 mg/day) or placebo. Before and after treatment, BAT volume and activity (PET-CT scans), resting energy expenditure (indirect calorimetry), body weight and body composition (DEXA scan) was determined. Furthermore, before and after treatment, blood samples were drawn to measure plasma lipids and glucose. An oral glucose tolerance test was performed to assess glucose tolerance and a skeletal muscle biopsy was taken.
Twelve weeks of Sitagliptin was well-tolerated and improved glucose tolerance as evident from an improved response to an oral glucose load. In addition, Sitagliptin lowered serum lipid levels. These effects were not accompanied by changes in body weight, body composition and energy expenditure. Also, BAT volume and activity as indicated by glucose uptake by BAT were not different.
In conclusion, we showed that twelve weeks of Sitagliptin in overweight prediabetic Caucasian men improved glucose tolerance and the lipid profile, without affecting energy expenditure or glucose uptake by BAT. Whether Sitagliptin could also prevent or delay the progression of prediabetes into full-blown type 2 diabetes or the development of dyslipidemia in these individuals at risk for developing metabolic disease has to be further explored in future studies.


