Biomarkers for early detection of NASH-induced fibrosis in humans
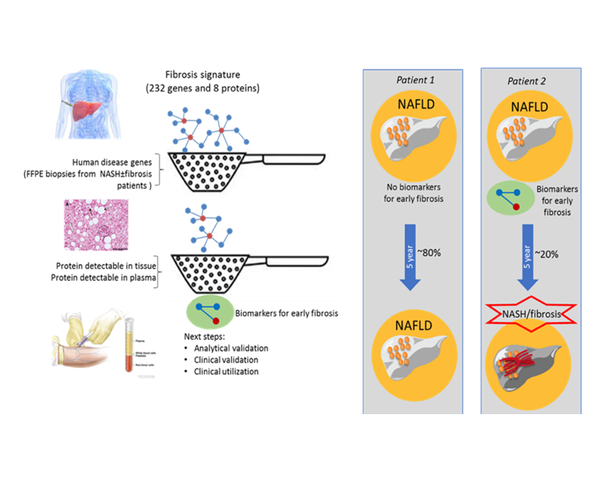
The purpose of this project is to develop a blood-based biomarker panel for the detection of non-alcoholic steatohepatitis (NASH) induced fibrosis. The projects builds upon previous (pre-clinical) work which defined a set of candidate biomarker set in mice. The aim of this project is to verify this set of candidates in human and translate this set of biomarkers into a diagnostic tool for stratification of NASH patients. A public-private partnership was established between TNO, Takeda, GenomeScan, Good Biomarker Sciences, Erasmus MC, AMC and UMC Utrecht.
Together with the prevalence of obesity the risk for developing NASH is increasing in western population. NASH is characterised by hepatocellular damage and inflammation, which drive the development of fibrosis. Liver fibrosis is strongly associated with long-term overall mortality. Clinical symptoms of liver fibrosis become manifest at an advanced stage of disease and early diagnosis is not possible. Moreover, it is not yet possible to stratify patients at risk, to be able to select the 25% of patients which develop active fibrosis. Therefore there is a need for novel biomarkers that can determine the early onset of liver fibrosis.
In short, first evaluation takes place in silico which candidate biomarkers are published in current literature. This list is completed using with in-house multi-omics data from a wide range of (pre-) clinical studies. Next, these markers that are present in human Formalin-Fixed Paraffin Embedded (FFPE) liver biopsies are verified. A ranked set of most relevant, mechanism based biomarkers will be selected to be evaluated in human plasma to enable stratification. The ultimate deliverable will be a blood-based biomarker assay and/or algorithm detection of NASH-induced fibrosis in patients.
This collaboration project is co-funded by the PPP Allowance made available by Health~Holland, Top Sector Life Sciences & Health, to TNO to stimulate public-private partnerships. For questions, please contact TNO directly via the following email address hans.hennekam@tno.nl.







