A novel low-risk approach to improve treatment of hematopoietic disorders
Hematopoietic stem cell transplantation (HSCT), to treat inherited blood diseases such as sickle cell anaemia and primary immunodeficiencies including X-linked severe combined immunodeficiency (SCID), has proven to be effective for the treatment of hematopoietic disorders. However, current preparation for HSCT carries significant toxicity due to radiation or chemotherapy that put the patient, often at very young age, at high risk of life-threatening infections and toxicity. Therefore, Erasmus MC and Harbour Biomed will develop a low-risk solution by engineering and pre-clinically testing of antibodies that rapidly and transiently prepare the bone marrow of patients for HSCT without toxicity.
The improved HSCT protocol will enhance the quality of life of a large group of patients. This novel approach is also needed to make the upcoming clinically safe genome editing tools that are currently in development for the generation of genetically corrected autologous hematopoietic stem cells successful. In 2030, the share of people with a chronic illness that prefers to participate in society is increased by 25%. In addition, the life-long health care cost for primary immunodeficiency patients has been estimated to be $55,000/year. Developing a safe method for corrective autologous HSCT is expected to significantly reduce these costs and greatly reduce comorbidities. Because of reduced hospitalisations patients will be able to attend school and work. Thus health-related productivity losses would be greatly reduced.
The consortium will develop and optimise novel antibodies to improve bone marrow conditioning prior to HSCT. These antibodies will be thoroughly tested in the laboratories for proper functionality. Completion of these studies will poise to be ready to test a novel approach in clinical studies.
At the end of the project, the consortium expects to have an optimal low-risk antibody-mediated method for bone marrow conditioning prior to HSCT. Next, this antibody will be produced by Harbour Biomed for clinical usage.
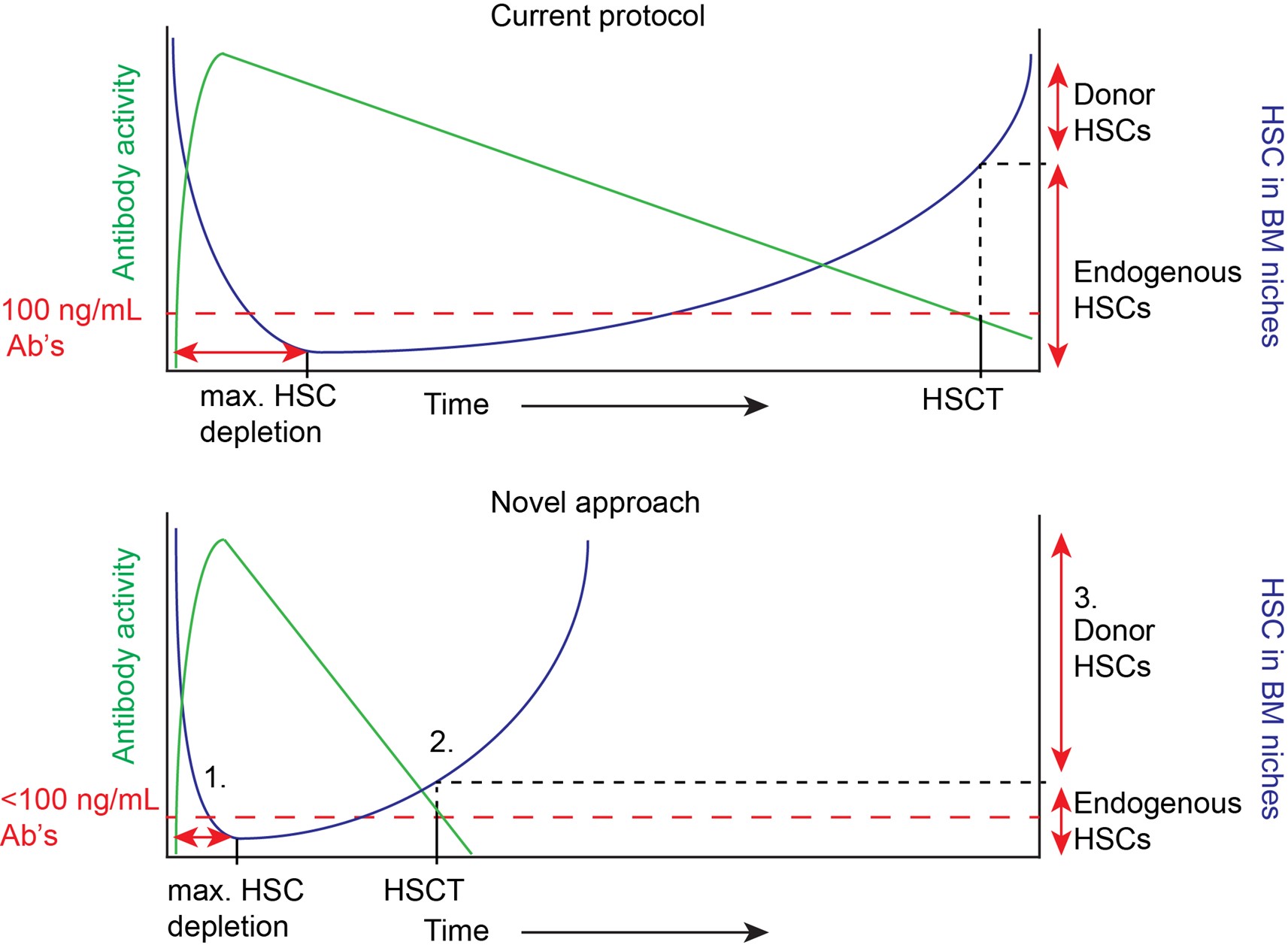
Schematic overview of the kinetics of current c-KIT antibody-mediated depletion of HSCs and our novel approach. We expect to improve the current HSCT protocol in three ways: 1. Due to improved activity of HSC-eliminating antibodies the maximum depletion of HSCs will be reached at earlier time-point. Also, the concentration limit, which will be different per antibody, will be reduced. 2. Due to the short half-life of the c-KIT antibodies, patients can be transplanted at an earlier time-point with less interfering antibodies in the host leading to improved engraftment. 3. Due to the optimized kinetics, we expect to reach higher percentages of donor chimerism after HSCT. Green: Antibody activity, Blue: host HSC in BM niches, Red: concentration limit Ab’s for HSC transplantation


