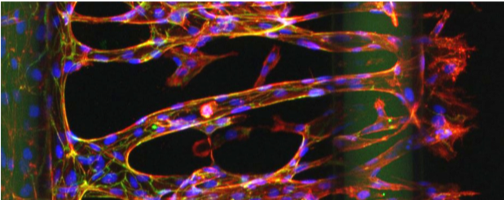
Growing real human small blood vessels in the laboratorium
In this project, basic scientists of the Leiden based LUMC and the LACDR will work in close collaboration with SMEs Mimetas and Ncardia to develop a next generation capillary-on-a-chip model to study the mechanism of small blood vessel loss in disease such as ‘heart failure with preserved ejection fraction’(HFpEF). In patients with HFpEF a progressive loss of perfusion in the heart muscle ultimately results in a reduction of the pump function of the heart. For the estimated 65.000 HFpEF patients in the Netherlands therapeutic options are severely limited. Notwithstanding the clinical relevance of small vessel disease, its underlying mechanism is poorly understood as current in vitro models for human small vessels lack sufficient complexity to assess the functionality of human capillary endothelial-pericyte and matrix interactions. As a result, therapeutic options to counteract rarefaction are virtually non-existent.
They recently developed a microfluidics-based, 3D microvessel-on-a-chip’ platform that allows the formation of 10- 30 µm wide human capillaries including perfusable lumen. This model is unique in that it combines the use of MIMETAS OrganoPlate that allows balancing of complexity and throughput in a physiologically relevant cellular microenvironment with Ncardia’s hiPSC-derived ECs which resemble primary cells that can be expanded in nearly unlimited quantities scalable and are amendable to genetic editing. However, the final step in the modeling of true physiological capillaries is the introduction of capillary-associated pericytes.
In this project they optimized co-culture conditions for iPSC-derived EC and pericytes/vascular smooth muscle cells (VSMC) and provided proof of principle that the application of perfusion associated shear-stress on our capillary networks can positively affect the pericyte-capillary EC interaction to the extent of physiologically relevant interactions. This “breakthrough” finding has allowed to now optimize the robustness of the generation of the pericyte stabilized capillaries and they strongly believe that our pericyte-stabilized capillary-on-a-chip platform will provide an essential tool to interrogate how blood plasma factors drive capillary rarefaction in patients and
ultimately may contribute to the development of therapeutic options to counteract microvascular associated cardiovascular disease.
More information can be found on these website:
- http://www.einthovenlaboratory.com/medewerkers/vincent-van-duinen-msc/
- https://www.universiteitleiden.nl/en/staffmembers/thomas-hankemeier#tab-1
- https://mimetas.com
- https://www.ncardia.com/