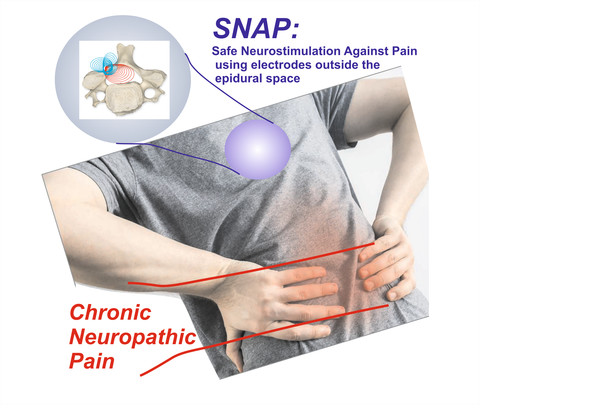
Safe Neurostimulation Against Pain
In 2018, a public-private partnership was established between the Department of Medical Technology of the University Medical Center Utrecht, and the company ELANA (Utrecht, the Netherlands), in order to develop a new electrode implant against chronic neuropathic pain, which, in contrast to the currently clinically used epidural catheter, can be placed safely outside the vulnerable epidural space.
Chronic neuropathic pain causes a dramatic loss of Quality-of-Life in many patients, and often can only be treated using medications (such as morphine) that have serious side-effects. Therefore, many efforts have been taken to find an alternative. The most successful alternative is direct electrostimulation of the spinal cord (SCS). It has been shown that stimulation of the dorsal columns in the spinal cord can block the transmission of pain signals to the brain. For example, in patients with neuropathic leg pain, 48% of the patients experienced substantial pain relief after 6 months of SCS, compared to 9% when using only conventional medication. However, the current clinical SCS technique uses electrodes on a catheter that needs to be placed within the very vulnerable epidural space, which is a major drawback (risk of infection or connective tissue formation).
To overcome these problems with the existing SCS technique, the consortium partners formulated a new concept (SNAP, Safe Neurostimulation Against Pain), in which the electrodes are placed OUTSIDE the vulnerable epidural space. In order to make this work, they developed a new multi-electrode approach and a wire-shaped implant that takes advantage of the electrically insulating property of the bone tissue of the spinal cord as a means of projecting the electrical stimulation current into the target area in the spinal cord.
Upon completion of the project, the efficacy of the SNAP system will have been tested in a realistic in vitro set-up using post-mortem human tissues.