CRISPR-Cas9: from a molecular scissor to a surgical knife
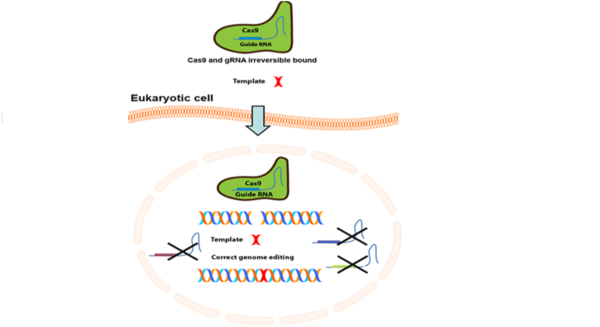
In collaboration with our commercial partner Biolegio, Erasmus MC aims to develop a DNA repair tool in which the molecular scissor (Cas9), a protein that is used to cut DNA, is irreversible bound to its CRISPR, a guideRNA that allows Cas9 to find and repair, i.e., disease causing mutation. Our combined effort will allow us to transform the molecular scissor Cas9 in a surgical knife.
Recent work at the Erasmus MC revealed that when Cas9 is unguided, severe DNA damage is introduced in the human cell. This finding lead to the idea that by locking the CRISPR guideRNA in Cas9 the DNA damage can be prevented during the repair of disease causing mutations.
Within this project, the partners investigate the possibility to irreversibly bind the CRISPR guideRNA in Cas9, by chemical modifications. The CRISPR-Cas9 genome editing technology is widely used for research purposes and explored for clinical applications, but the ability of Cas9 to induce DNA damage was relatively unknown. Therefore no techniques are available at the moment to circumvent this problem.
This project will result in solid data, for the technical aspect, namely the irreversible binding of the CRISPR to Cas9. When successful, our method provides the foundation to reveal whether the CRISPR-Cas9 DNA repair technology can be brought a step closer to the clinic by analysing the safety requirements that are needed according to the European Medicines Agency.