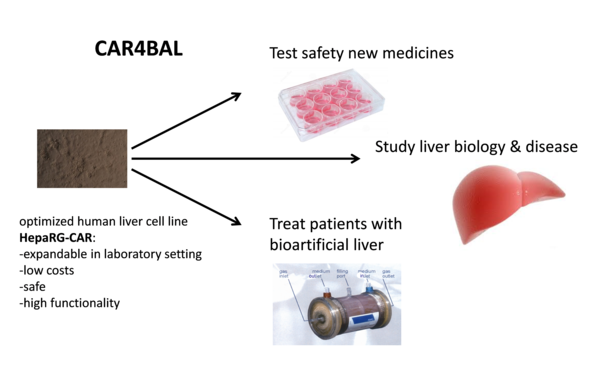
Creating an optimal source for human liver cells
In the CAR4BAL project the Academic Medical Center (AMC) has joined forces with the companies Hep-Art Medical Devices B.V. and Biopredic International in a public-private partnership to generate a source for safe and highly functional human liver cells.
Human liver cells are highly needed for testing medicine toxicity to the liver, scientific research to combat liver diseases and infections, like malaria, and as biocomponent in bioartificial livers (BALs). BALs consist of bioreactors with liver cells that are temporarily connected to liver failure patients for liver support. The currently applied human liver cells are either functional, but scarce, or highly available, but low in functionality, and therefore the medicine safety testing, and scientific research of liver diseases are not optimal and BALs are still not available. Currently this costs many lives. For instance, liver toxicity of prescribed medicines is responsible for 5% of all hospital admissions and 50% of all acute liver failures.
The CAR4BAL project will produce a new cell line with DNA encoding a regulator of liver maturation, the constitutive androstane receptor (CAR) at a defined position in the DNA of the human liver cell line HepaRG. The performance of the new HepaRG-CAR cell line will be optimised and evaluated for drug safety testing and BAL application. Furthermore regulatory demands for clinical BAL application will be evaluated, and a commercialisation strategy will be delineated.
During the CAR4BAL project it was decided not to generate a HepaRG-CAR cell line with the CAR-gene at a defined position in the genome, but to continue with the existing HeparG-car cell line, to limit the risks and delays. This HepaRG-CAR line was further analyzed for functionality, safety, stability and growth characteristics and compared with its parental HepaRG cell line. The HepaRG-CAR line showed improved safety, functionality and economic profile (lower medium costs and higher stability), which establishes a foundation for exploiting HepaRG-CAR cells as source for human liver cells. A preliminary set-up of the production process of BALs loaded with HepaRG-CAR cells was established. The business plan for exploitation of the HepaRG-CAR cells is in development. The partners aim to bring this cell line further to the market.
In conclusion, the CAR4BAL results provide solid evidence for the high potential of HepaRG-CAR cells in improving applications requiring human liver cells, and, as a consequence, the health-related quality of life of citizens, and in strengthening the position of the involved companies and their academic partner.