Antiretroviral agents in HIV-infected pregnant women
HIV-infected pregnant women need to be treated with antiretrovirals during pregnancy to prevent the transmission of the virus to the child. The body changes in pregnancy, due to which standard dosing may not be adequate in pregnancy. Because research in pregnancy is ethically challenging, we explore alternative ways to study the behavior of antiretrovirals in pregnancy. A better understanding of the mechanisms underlying absorption, distribution, metabolism and excretion of antiretrovirals in pregnancy can aid in designing rational dosing adjustments. Specific interest relates to fetal antiretroviral exposure, because of toxicity. This is of interest for pharmaceutical industries responsible for marketing of such drugs, but also clinicians who need to prescribe the medicine to their patients.
There are currently no evidence-based recommendations for dosing of antiretroviral agents in pregnancy. Label information of the majority of antiretroviral drugs discourages the use in pregnancy, due to lack of data. It is left upon the discretion of the physician to prescribe the medication and to adjust the dose or not. The fact that 25% of the pregnant women in The Netherlands had a detectable viral load (>50 copies/mL blood) at delivery, despite antiretroviral treatment, might indicate that standard dosing during pregnancy is sub-therapeutic.
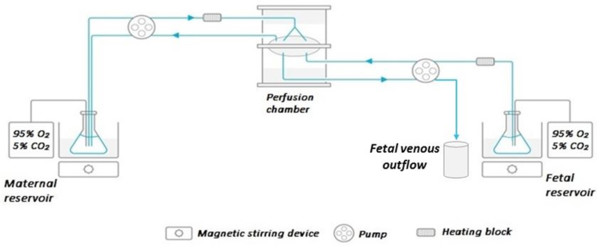
Physiologically-based modelling (in silico) may provide a solution since this approach is not based on clinical data, but rather in vitro and ex vivo data. This makes it a valuable tool to simulate maternal and fetal exposure to antiretroviral agents in pregnancy, without exposing pregnant women to a drug under development. In this project, in vitro metabolism and transport assays are used, but also ex vivo perfusion of isolated placenta’s. This data is integrated in physiology-based pharmacokinetic models to predict behavior of antiretroviral drugs in pregnant women and the fetus.
Eventually this will lead to models that can be used to investigate optimal dosing strategies in pregnancy. This has been, and will be communicated in the form of scientific publications and presentations at international conferences. Treatment guidelines include placental perfusion data, we strive to have our data included. This knowledge will help build a more advanced understanding of pregnancy-related pharmacology in general.
Janssen Research and Merck provided input for the PBPK models, Janssen Research use the darunavir model as developed by the Radboudumc for their modelling.
Fig. 1: Schematic of the placenta perfusion set-up