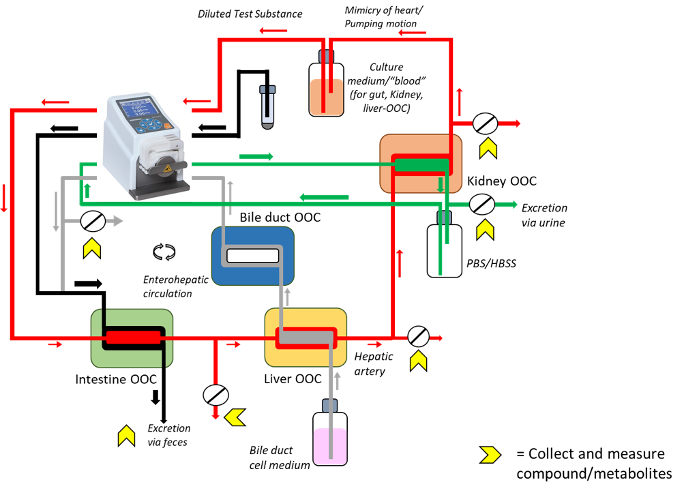
Combining liver, kidney and gut-on-chip to predict human pharmacokinetics in vitro
Each year, tens of thousands of compounds are studied by the pharmaceutical industry. Lack of efficacy and safety are the most common reason for drug failure during development (>90% in expensive clinical phase studies). Drug disposition is tested by studying drugs’ pharmacokinetics (PK; the fate of a compound in the body), which can be very different between laboratory animals and human beings.
The US Food and Drug Administration Agency and The National Centre for the 3Rs (reduction, refinement and replacement of animal studies; NC3Rs UK) estimated that yearly 0.2-1.5 million animals are used by the pharmaceutical industry for PK-testing. Currently available and applied cell-line based models also have limitations in predicting human drug absorption, distribution, metabolism and excretion properties (comprising PK), underscoring the necessity for improved preclinical models. In the last decade, two exciting developments occurred in the in vitro field: (1) The 3D-culture of mini-organs from human cells that physiologically mimic human organs, and (2) the organ-on-chip technology, being a cell source subjected to a very small fluid stream within a 3D-fabricated structure representing the human body fluid dynamics.
Here, the proposition is to use these developments to design and construct a system that connects different organ model systems, thereby mimicking medication uptake, chemistry and removal. After entering the body from the gastro-intestinal tract, the drug compound is distributed throughout the body, either unchanged or modified in the liver and excreted from the body via the liver through the bile duct and/or the kidneys into urine. These processes will be replicated in our state-of-the-art model system, comprising four organ-chips. The Drug Disposition-on-a-Chip is a potential game-changer that can both replace animal experiments and can greatly advance drug development. The close collaboration with industrial partners will ensure rapid implementation and open up new economic perspectives.