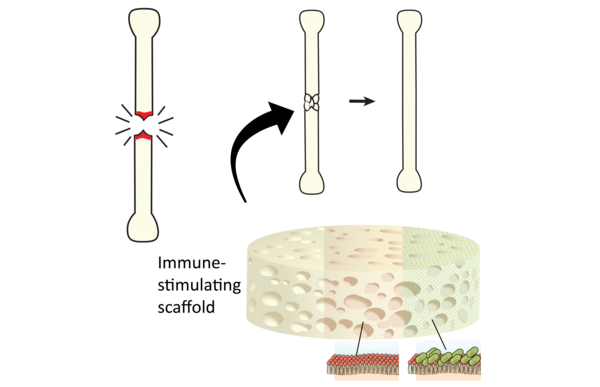
iBone: triggering the immune response to heal bones
There currently is no satisfactory alternative for the widespread use of bone transplantations to heal bone defects.
The iBone project is a collaborative effort with Kuros Biosciences to maximise the potential of the latest generation bone substitutes.
Failure of the natural bone healing mechanism occurs in 10% of trauma and orthopaedic surgeries. This necessitates additional bone reconstruction techniques to boost healing. The most applied strategy is to transplant patient’s own bone, however this has serious drawbacks in terms of safety and efficacy. In addition, the increased hospital stay and extended operation time increases the costs by €2000-3000 per patient. Spinal fusion surgeries account for more than half of the bone grafting applications, which equals 3% of all operating room procedures.
iBone aims to instigate bone healing with a controlled inflammatory response. The basis for this idea is the clinical observation that certain infectious or inflammatory diseases lead to huge amounts of new bone formation. The goal is to develop an enhanced bone construct based on the most optimal synthetic carrier that is available to date, augmented with immune-modulatory, bone-inductive stimuli. At the end of this project, the consortium aims to have a product with proven efficacy in a challenging pre-clinical scenario.
Specific immune-stimulatory components have already been identified that lead to enhanced bone formation in small animal models. In the iBone project, large comparative studies will be performed to identify immune-stimulating compounds with optimal bone-inductive properties. Coating will be developed to maximize their immunogenic and bone-inductive properties. In a final stage, the subset of novel deliverables will be tested in direct comparison to the current gold standard in large preclinical bone healing models such as spinal fusion.